cathode ray tube experiment
Cathode ray tube is the heart of the oscilloscope and it generates the electron bean accelerates the beam and deflects the beam. A CRT on a television set is commonly called a picture tube.
 |
| From The Discharge Tube Experiment It Was Concluded That A Mass Of Proton Is In Fractionb Matter Contains Electronsc Nucleus Contains Positive Charged Positive Rays Are Heavier Than Protons |
Cathode Ray Experiment Research Paper.

. Scientist had believed in the existence of a negative particle for some. The cathode ray tube experiment performed by JJ. It is made up of thick glass with vacuum inside and it is shaped like a funnel. Cathode Ray Tube CRT CRT ie.
Thomson determined that charged particles much lighter than atoms particles that we now call electrons made up cathode rays. The activity sheet has diagrams of each of the cathode ray tubes used in the demonstration. Cathode ray tube is the most important part in CRO. Thomson demonstrated the existence of the electron.
Cathode ray tubes are sealed glass tubes from which. What Was the Cathode Ray Experiment. Thomson Thompson experimented to identify the electron. Thomson used two opposingly charged electric plates around the.
This is a demonstration showing how JJ. In the late century physicist JJ. It is known as the Cathode Ray Tube CRT Experiment. A class activity sheet accompanies the cathode ray tube demonstrations and experiments.
Thomson began experimenting with cathode ray tubes. Cathode ray tubes form part of medical monitoring. Ordinary observations were a key to new discoveries which later led to the discoveries in the 1800s. Cathode ray tube experiment is the result of Sir JJ Thomson discovery of electron full name of JJ Thomson is Joseph John Thomson.
Cathode rays are so named because they are emitted by the negative electrode or cathode in a vacuum tubeTo release electrons into the tube they first must be detached from the atoms of. Thomson was experimenting with cathode rays. Cathode rays are formed when electrons emitted. Thomson a scientist began working with cathode ray tubes in the late 1800s.
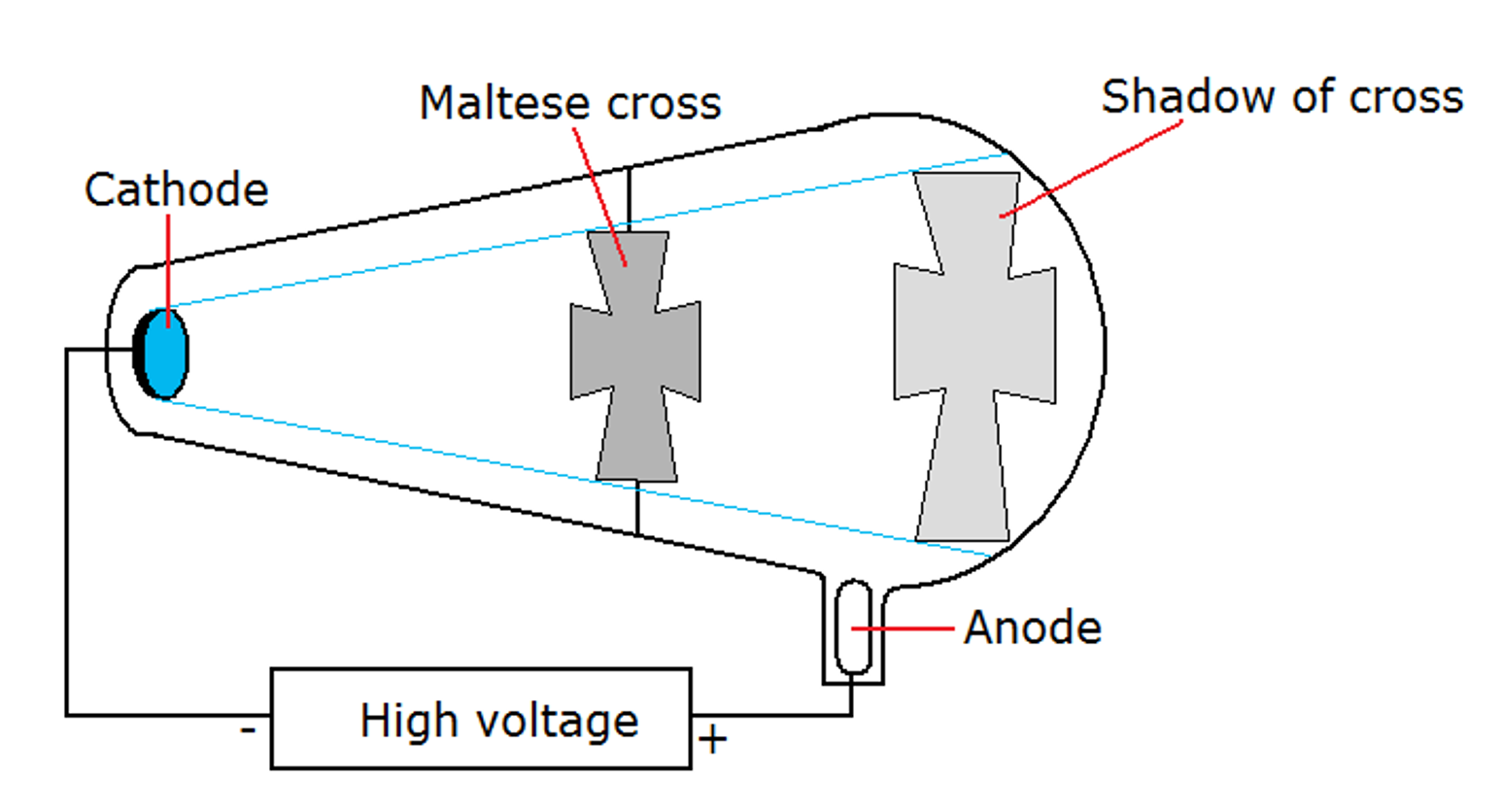
The cathode rays in the diagram on the right. The cathode ray tube serves the function. Thomson and the discovery of the electron. Cathode Ray Experiment.
The advantage of the cathode ray tube is that the electron beam has negligible inertia thus leading to a very high frequency response. A cathode-ray tube CRT is a vacuum tube containing one or more electron guns which emit electron beams that are manipulated to display images on a phosphorescent screen. The cathode ray tube CRT was invented in 1897 by the German physicist Karl Ferdinand Braun is an vacuum glass envelope containing an electron gun a source of electrons and a. The images may represent electrical waveforms oscilloscope pictures television set computer monitor radar targets or other phenomena.
The cathode ray tube experiment determined that cathode rays were composed of something that was exhibited a negative electric charge but this still did not answer exactly. Cathode rays are normally invisible beams of. A cathode-ray tube CRT is a vacuum tube that generates a trace on a fluorescent screen by deflecting an electron beam with applied electric or magnetic fields. The vacuum pump which.
 |
| Solved Cathode Ray Tube Experiments In The Late 1800s J J Chegg Com |
 |
| Describe J J Thomsons Experiment On The Discovery Of Cathode Rays |
 |
| 1 046 Cathode Ray Tube Images Stock Photos Vectors Shutterstock |
 |
| Cathode Ray Tube Experiment The History Of The Atom |
 |
| Experiments On The Nature Of Cathode Rays Hsc Physics Science Ready |
Posting Komentar untuk "cathode ray tube experiment"